Description
Simplify Your Sri Lanka National Medicines Regulatory Authority Re-Registration Process
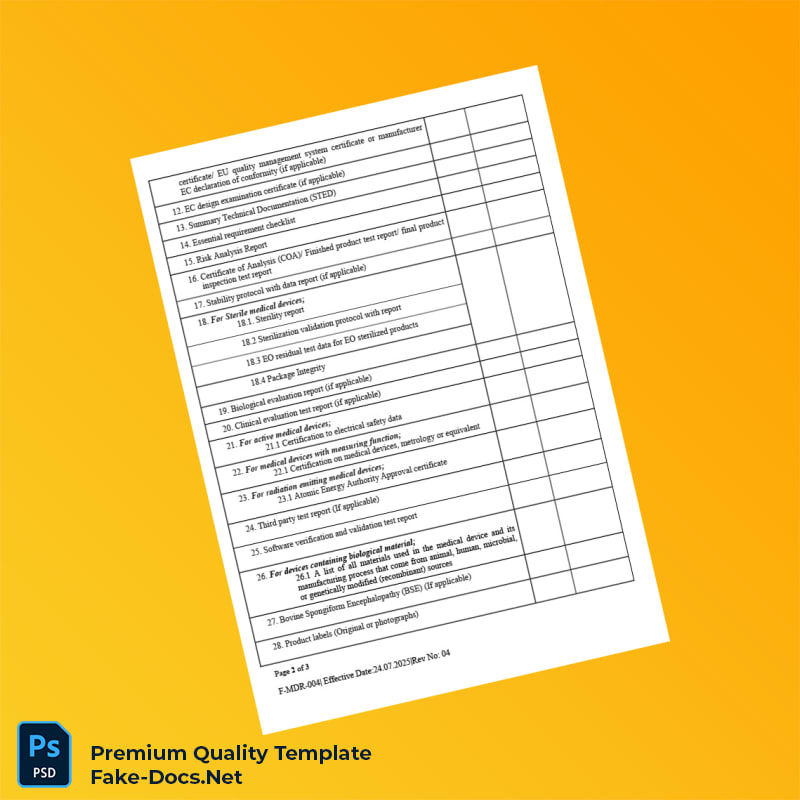
Managing the complex requirements for regulatory document submissions can be daunting for pharmaceutical professionals and regulatory affairs specialists in Sri Lanka. The Sri Lanka National Medicines Regulatory Authority Re-Registration Dossier Submission Checklist Template (Word & PDF) offers a streamlined solution designed to facilitate a seamless dossier preparation experience. This editable template ensures every vital detail is accounted for efficiently, minimizing delays and compliance risks.
Efficient Documentation Management Tailored for Regulatory Needs
Imagine a regulatory officer who struggled with compiling scattered re-registration documents, facing repeated resubmissions. By leveraging this comprehensive checklist template, available in both editable Word and secure PDF formats, they organized their entire dossier effortlessly, satisfying all submission criteria the first time. Our template integrates flawlessly with other essential documents found under registration certificates, creating an all-encompassing document management system.
Precision and Convenience with Editable Features
Every document detail—from applicant information to regulatory references—is editable, allowing for timely updates without the need to recreate files. The template is optimized for instant download, includes high-resolution formatting for professional printing, and encompasses comprehensive instructions and predefined fields to ensure compliance with the Sri Lankan regulatory framework. You will benefit from enhanced accuracy and time savings, crucial when meeting strict submission deadlines.
Designed for Regulatory Professionals and Compliance Teams
This checklist is an indispensable asset for pharmaceutical companies, quality assurance teams, and regulatory affairs consultants who demand reliability and clarity in regulatory documentation. It mitigates common issues such as omissions, inconsistent data, or formatting errors that cause costly processing delays. By using this template, professionals experience improved document control and a higher probability of expedited approval.
Get Started Quickly and Protect Your Regulatory Success
Don’t let administrative challenges hinder your regulatory commitments. Access the Sri Lanka National Medicines Regulatory Authority Re-Registration Dossier Submission Checklist Template today and secure a professional, compliant dossier submission. Streamline your process, reduce errors, and increase confidence in meeting regulatory benchmarks. Download now and transform your registration workflow efficiently.










Reviews
There are no reviews yet.